Publications Summary
Dr. Gradinaru’s research group at Caltech specializes in developing neuroscience tools and methods, including engineering of new viral vectors with optimized brain tropism after systemic delivery. In addition to developing technologies for neuroscience, Dr. Gradinaru has also been using such tools and methods to dissect circuitry underlying movement, mood, and sleep disorders (Gradinaru et al., Science, 2009; Xiao et al, Neuron, 2016; Cho et al, Neuron, 2017; Oikonomou et al, Neuron, 2019). The Gradinaru group at Caltech has recently developed and disseminated various new tools for less invasive gene delivery and optogenetics to the brain (Deverman et al Nature Biotechnology 2016; Chan et al Nature Neuroscience 2017; Challis and Kumar et al Nature Protocols 2019; Bedbrook et al Nature Methods 2020; Kumar et al Nature Methods 2020). With collaborators and her own Caltech group, Dr. Gradinaru is now applying these gene delivery tools to neurodevelopmental and neurodegenerative disorders (Challis et al Nature Neuroscience 2019; Rauch et al Nature 2020).
Expanding the brain researcher’s toolkit
Despite the wealth and quality of basic neuroscience research, there is still little we can do to treat or prevent most brain disorders. Industry efforts, meanwhile, have shied away from this field, particularly after a series of major drug candidates for the treatment of Alzheimer’s disease failed to meet expectations (1).
My previous research, which entailed developing and using optogenetics (2, 3) to understand how deep brain stimulation works in Parkinson’s disease (PD) (4, 5), resulted in two key insights: We need to look and intervene earlier in brain disease progression, and we need to be able to access relevant cell populations with noninvasive yet precise tools to investigate, prevent, contain, or even reverse the course of disease. Accumulating evidence has highlighted a third insight: We may need to look beyond the brain to fully understand brain disorders (6, 7).
My goal has been to develop an effective toolkit for neuromodulation so we can start to bridge the gap between what we know and what we can do to treat the brain. To achieve minimally invasive optogenetic-mediated modulation, we need to be able to penetrate the blood–brain barrier (BBB) so that vectors can be delivered systemically rather than through intracranial injections and address the poor reach of visible light through tissue so that large tissue volumes can be recruited without implantation of optical fibers. For early intervention, we need to get past the neuronal and brain-centric view of neurological disease.
Penetrating the Blood–Brain Barrier
The adult brain is protected from compounds circulating in the blood by the BBB. Gene delivery to the brain requires surgery that is not only invasive but also results in limited tissue coverage and nonuniform gene expression.To achieve sufficient coverage for conditions characterized by broad dysfunction, such as neurodegeneration, multiple injections are needed, each of which creates local inflammation and damage. Systemic delivery would therefore be preferable to focal delivery because it does not require surgery and achieves broader tissue coverage.We have pioneered a powerful strategy that allows for the generation and selection of adeno-associated viral (AAV) vectors with optimized properties through Cre-recombination-dependent AAV-targeted evolution (CREATE) (8). My lab has used CREATE to synthesize AAVs that cross the BBB and transduce most cells in the adult mouse brain (see the first figure). These systemically delivered AAVs enable noninvasive brainwide transduction of specific cell types and regions in rodents when used with gene regulatory elements (9).
Developing Opsins for Minimally Invasive Recruitment of Large Tissue Volumes
During our quest to achieve systemic delivery of AAVs with opsin cargoes, we learned that the lower per-cell transgene copy number produced by systemic delivery led to ineffective overall opsin conductance. We needed better, high-performance opsins to make this method viable. To achieve such opsins, we built diversity into channelrhodopsin (ChR) using structure-guided SCHEMA (10) protein recombination from distinct opsin parents and then measured membrane localization and photocurrents. However, the dominant method for testing opsin properties—whole-cell patch clamping—has low throughput, so we used machine learning with limited training data to efficiently explore the vast sequence space and restrict the number of opsins to be tested.
With Gaussian-process models trained on a limited experimental set of 102 functionally characterized ChRs, we selected ChR sequences that the models predicted would express, localize, and function. The result was a panel of high-photocurrent ChRs with exceptional light sensitivity (ChRgers) (11).
These high-fluxing opsins not only overcome the low–copy number limitation of systemic delivery but also allow the light source to be placed at a distance from the transduced cells (for example, on a thinned mouse skull rather than implanted directly in the brain).
The net effect of these advances is more effective coverage by both light and transgenes. This may be particularly useful for advancing optogenetic studies in nonhuman primates (NHPs), a key model relevant to human health. Optogenetics has had a relatively limited impact in NHP research, compared to its impact in smaller model organisms, predominantly owing to coverage problems stemming from the delivery limitations for genes and photons. Using systemic delivery of high-performance opsins to transduce and recruit large brain regions in NHPs could enable better neuromodulation in these important animal models.
Overcoming the Neuronal and Brain-centric View of Neurological Disease
Neurodegeneration research has focused mainly on compromised neurons and circuits in the brain. Nevertheless, evidence points to roles for inflammation mediated by non-neuronal brain cells and body-to-brain connections (by way of the peripheral nervous system and/or a compromised BBB) (6, 7).
Engineering gene-delivery tools that specifically target non-neuronal brain cells relevant to neurodegeneration, such as the brain endothelial cells that constitute the vasculature and the BBB, may be paradigm shifting because an impaired BBB can initiate and/or precipitate neurodegeneration. The ability to perform both positive and negative selection is key to yielding vectors with desired organ, cell type, and cell region tropisms.
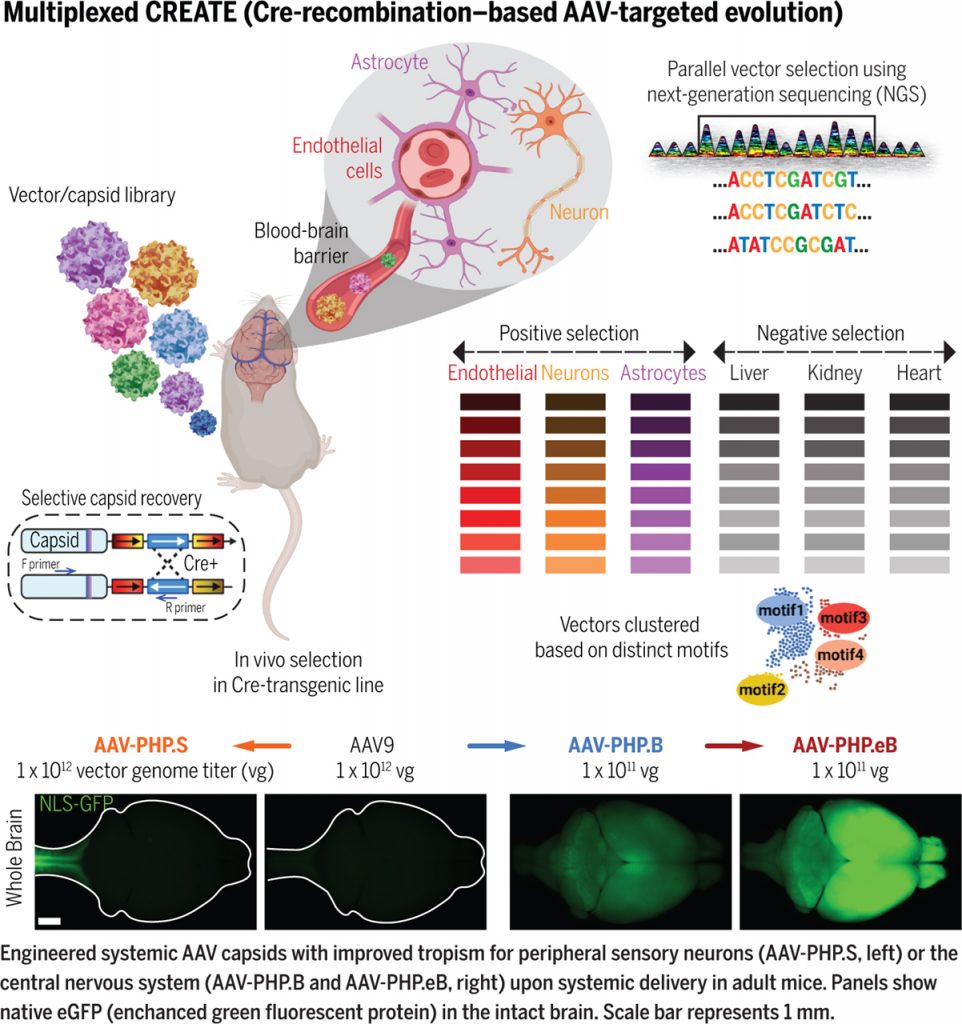
To enable this, we transformed our CREATE platform into multiplexed M-CREATE (12), an in vivo screening strategy that incorporates next-generation sequencing, synthetic library generation, and a dedicated analysis pipeline. Using M-CREATE, we identified capsid variants that exhibited bias toward vascular cells or that targeted neurons with greater specificity, as well as capsids that transduced the central nervous system broadly or crossed the BBB in diverse murine strains.
As a weak BBB can allow pathological factors into the brain (13, 14), functionally targeting BBB permeability by means of engineered AAVs can affect body-to-brain access through the circulation. Modulating permeability affords the opportunity to study and/or repair a barrier that can be weakened in disease or to deliver therapies to the brain by way of the bloodstream.
Modulating the Peripheral Nervous System to Address Central Dysfunction
Synucleinopathies are neurodegenerative diseases characterized by the aggregation of insoluble amyloid α-synuclein (αSyn) fibrils. PD is a synucleinopathy characterized by death of selected midbrain and brainstem neuronal populations and motor dysfunction. Roughly 90% of Parkinson’s cases arise sporadically, making the study of its etiology difficult. Emerging findings suggest that nonmotor features such as loss of smell and gastrointestinal deficits may precede clinical diagnosis (15, 16).
Postmortem biopsies from asymptomatic PD-diagnosed individuals have revealed the presence of pathologic αSyn assemblies in gastrointestinal tissue, leading Braak and colleagues to suggest that αSyn aggregation may originate in peripheral tissues such as the gut and progress to the brain by way of autonomic fibers (17, 18). Understanding the role of the peripheral nervous system in propagating pathology may therefore aid our understanding of neurodegeneration and help prevent it.
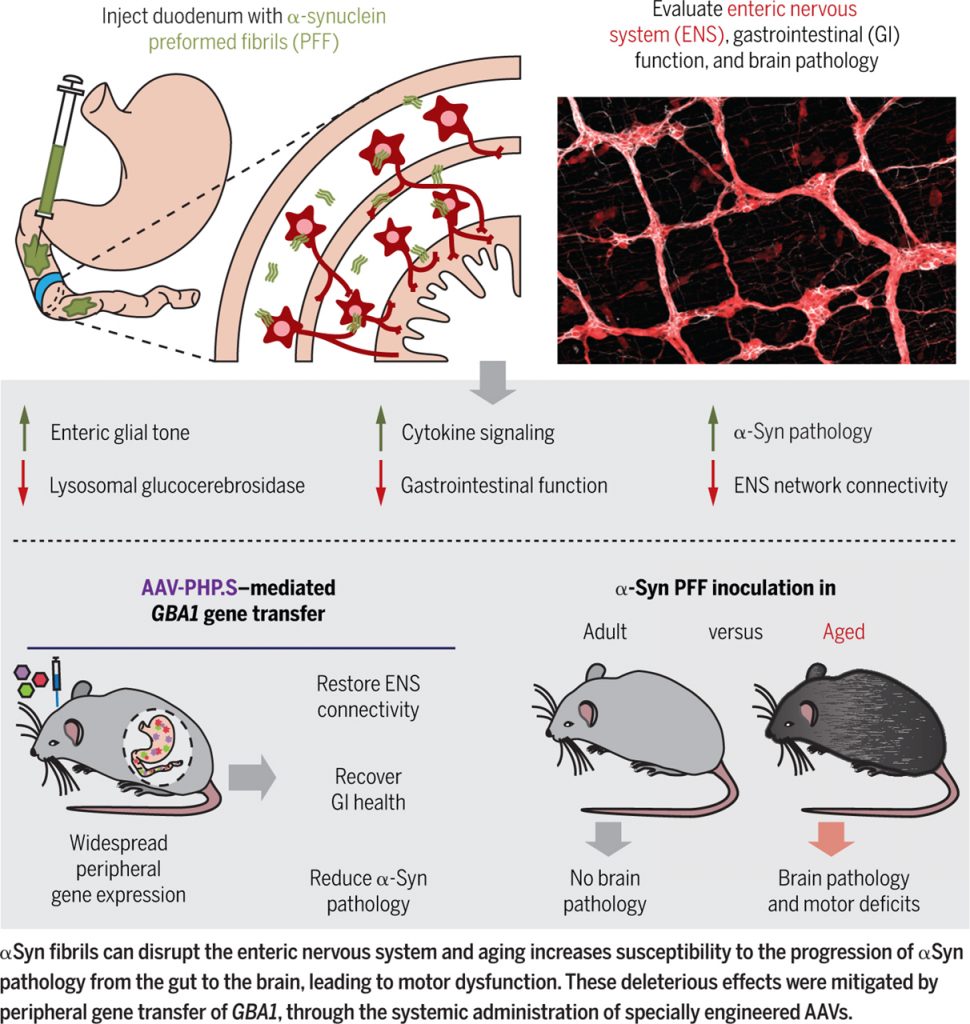
Recognizing the lack of tools and methods available to study peripheral nervous systems, we developed whole-body tissue clearing and a tunable and rapid vector expression system that we used to evaluate network connectivity in the enteric nervous system (ENS) (19, 20). We injected a modest amount of αSyn preformed fibrils into the gut lining of mice (specifically the highly innervated duodenal wall) and observed subsequent gastrointestinal inflammation and physiological changes to the ENS (measured by optogenetics, calcium imaging, and changes in fecal production) (21). ENS pathology was also associated with a severe deficit in the lysosomal enzyme glucocerebrosidase, encoded by the gene GBA1, known to be involved in Gaucher disease and PD.
We therefore delivered GBA1 by means of the AAV-PHP.S capsid, which efficiently transduces the peripheral nervous system, to noninvasively restore glucocerebrosidase in the periphery. This led to a reduction in αSyn pathology and hints at a possible therapeutic target for early PD.
Lastly, we demonstrated that inoculation of αSyn fibrils in aged mice, but not younger mice, resulted in progression of αSyn histopathology to the midbrain and decreased dopamine in the striatum, and subsequent motor defects. Taken together, this work (summarized in the second figure) shifts the focus of neurodegenerative disease etiology to the peripheral nervous system and expands our understanding of the role played by the ENS in prodromal synucleinopathy.
Summary of Findings
We aim to bridge the gap between what is currently feasible in neuromodulation and what is needed to meaningfully improve the lives of those with neuropathologies. We have used protein engineering principles to noninvasively, effectively, and specifically deliver effector genomes to nervous tissues and associated cell types (22). We have made advances in the creation of systemic viruses that cross the BBB, which open up the potential for noninvasive modulation of targets deep in the brain. In addition, we have used our gene delivery and optogenetic tools to modulate the peripheral nervous system in a mouse model of PD, demonstrating the potential utility of neuromodulation beyond the brain for the treatment of brain disorders. A number of relevant barriers remain, including the need to expand the AAV modest packaging limit, to penetrate the BBB in a variety of species, and to carefully consider and mitigate AAV side effects with better vectors, delivery methods, and immune avoidance strategies. Nevertheless, the findings and resources generated by this work represent a step forward with implications for neurological disorders and will be generalizable across neurological and psychiatric indications.
Detailed Research Program
Financial support:
Work in the Gradinaru Laboratory at Caltech is funded by the following awards (to VG):
NIH: R01 NIA, NINDS BRAIN, R24&UC4 NIDDK, NIH Director’s New Innovator, PECASE, SPARC, Pioneer
NSF: Neuronex; DARPA
Foundations: CZI, Gates, Moore, Beckman, Curci, Sloan, Pew, Heritage Medical Foundation, ASAP